Lithium-ion batteries have revolutionized industries from mobile electronics and power tools to light electric vehicles, electric cars, and energy storage systems. However, despite their commercial success, safety concerns remain a major barrier to their broader application. Even in small consumer electronics, safety incidents involving lithium-ion batteries are still reported regularly. Ensuring battery safety is not only essential for user protection but is also a key prerequisite for the continued growth of the lithium battery industry.
Key Takeaways
-
The materials in lithium-ion batteries affect how safe they are. Picking the right ones can stop problems like fires or overheating.
-
Lithium iron phosphate (LFP) batteries are safer in high heat. They handle temperatures up to 275°C, making them useful for many things.
-
Solid-state electrolytes are a big improvement. They use solid stuff instead of flammable liquids, cutting fire risks and making batteries more stable.
-
New designs, like safety reinforced layers (SRL), have made explosions much less common. Explosion rates dropped from 63% to 10%, showing how better materials help safety.
-
Future studies focus on safer, non-flammable materials and recycling. These ideas aim to make batteries safer and help the planet too.
The Role of Safety Testing Standards
Cathode, anode, electrolyte, and separator
To protect both personal safety and property, comprehensive safety testing is essential for lithium-ion batteries. Over the years, a global framework of safety testing protocols has been established. The leading international bodies involved in lithium battery safety standards include:
-
Underwriters Laboratories (UL 1642)
-
United Nations (UN) Recommendations on the Transport of Dangerous Goods
Among these, only UL provides a formal UR certification mark for batteries that pass its standards. Many safety tests developed by other organizations are either adaptations or direct adoptions of UL’s testing procedures.
Key Categories of Safety Tests
Modern lithium-ion battery safety tests can be grouped into four main categories, each simulating potential abuse conditions that may occur during use, transport, or storage:
|
Group |
Main Tests |
|---|---|
|
Electrical |
Overcharge, overdischarge, high-temperature storage, external short circuit, forced discharge |
|
Mechanical |
Drop, impact, nail penetration, shock, crush, vibration, acceleration |
|
Thermal |
Flame exposure, hot plate, thermal shock, heat baths |
|
Environmental |
Decompression, high altitude, immersion, fungus resistance, high humidity/high temp tests (e.g., 4h@85°C) |
All lithium-ion battery safety tests are designed to simulate potential abuse conditions that may occur during transport, storage, or real-world usage. For example:
-
Nail penetration tests simulate internal short-circuit conditions.
-
Overcharge tests replicate scenarios where the protection circuit fails.
-
Environmental tests such as decompression and altitude simulations mimic conditions during air transportation or usage in high-altitude environments.
These tests are critical for identifying how a battery reacts under abnormal or extreme conditions, helping manufacturers evaluate and mitigate safety risks.
It is evident that safety tests vary significantly in difficulty. Environmental tests are generally easier for lithium-ion batteries to pass, and external short-circuit tests on small batteries also pose minimal challenge. However, certain tests are much more demanding—particularly those that cause the battery’s internal temperature to rise dramatically. As the internal temperature increases, it can trigger or accelerate chemical reactions within the battery. These reactions release more heat, causing the temperature to rise further. If this feedback loop continues unchecked, it can result in thermal runaway—a dangerous condition that may lead to fire, explosion, or catastrophic battery failure.
The most difficult safety tests typically include:
-
Overcharge tests without mechanical safeguards
-
Thermal oven exposure at 150°C for 120 minutes
-
External short-circuit tests
-
Nail penetration
-
Crush or impact tests (e.g., blunt force impact on cylindrical cells)
Battery Capacity: A Critical Factor in Safety Performance
Beyond the inherent difficulty of safety test protocols, battery capacity plays a crucial role in determining whether a lithium-ion cell can pass these tests. The larger the capacity, the more heat is generated and accumulated during abuse scenarios—greatly increasing the risk of safety failures such as thermal runaway.
Today’s commercial lithium-ion batteries used in consumer electronics are typically small and low in capacity. These batteries tend to pass safety tests more easily, and even when failures occur, the resulting damage or injury is usually limited. This relatively low-risk profile has supported their widespread adoption in mobile phones, laptops, and other portable devices.
In contrast, large-capacity lithium-ion batteries—such as those used in:
-
Battery Electric Vehicles (BEVs)
-
Plug-in Hybrid Electric Vehicles (PHEVs)
-
Hybrid Electric Vehicles (HEVs)
-
Electric motorcycles and scooters
face significantly greater challenges in meeting the same safety standards. The thermal and mechanical stress during testing is amplified by the battery’s size and energy density, making failure more likely and more dangerous.
Moreover, the consequences of a safety incident involving high-capacity batteries can be severe, including large-scale fires, explosions, equipment loss, and safety hazards to both users and facilities. This makes advanced safety design and rigorous testing not just important—but essential—in these applications.
Understanding Internal Reactions: The Root Cause of Lithium-Ion Battery Safety Risks

At its core, the safety challenges associated with lithium-ion batteries stem from internal heat generation and accumulation. If the heat produced during operation or abuse conditions cannot be efficiently dissipated, the battery’s internal temperature will rise uncontrollably. This rising temperature, in turn, accelerates electrochemical and side reactions inside the cell, ultimately triggering thermal runaway — a dangerous event that can lead to fire, explosion, or catastrophic failure.
Three Major Sources of Heat Inside a Lithium-Ion Battery
The internal heat generated during battery operation comes primarily from three sources:
-
Reversible Heat from Electrochemical Reactions
-
This is associated with entropy changes in the battery’s redox reactions.
-
Although this heat is part of normal energy conversion, it can contribute to overall temperature rise.
-
-
Irreversible Heat from Internal Resistance and Polarization
-
Includes ohmic losses and electrochemical polarization.
-
These losses are present even during standard charge-discharge cycles and increase under high current or aging conditions.
-
-
Heat from Side Reactions
-
This is the most critical in terms of safety.
-
Occurs when unwanted chemical reactions take place between battery components—often triggered by high temperature, overcharge, or internal damage.
-
These reactions are exothermic and can rapidly accelerate the temperature rise inside the cell.
-
Five Key Sources of Heat from Side Reactions (C-type Heat)
The third category—heat from side reactions—plays a central role in battery safety incidents. These exothermic reactions are primarily responsible for triggering thermal runaway. The five main contributors include:
Even under normal charge and discharge conditions, the first two sources of heat (A and B)—reversible and irreversible heat—are always present and generally manageable through thermal design and energy management systems. However, when heat from side reactions (Category C) is triggered, the situation can quickly escalate into a serious safety hazard.
The heat from side reactions originates from five primary mechanisms:
-
Decomposition of Cathode Materials
-
Certain cathode chemistries (e.g., NCM, NCA) release oxygen at elevated temperatures, which then oxidizes the electrolyte.
-
-
Electrolyte Oxidation at the Cathode
-
Under high voltage or elevated temperatures, organic solvents in the electrolyte may oxidize violently.
-
-
Thermal Decomposition of Electrolyte
-
Organic solvents (such as EC, DEC, DMC) begin to decompose, releasing flammable gases.
-
-
Decomposition of the SEI Layer on the Anode
-
The Solid Electrolyte Interphase(SEI), normally stable, breaks down under heat or overcharge conditions, leading to further reactivity.
-
-
Electrolyte Reduction at the Anode
-
High reactivity at the lithium or carbon anode surface can trigger reduction reactions with the electrolyte.
-
Due to significant differences in the onset conditions and heat generation associated with side reactions (Category C heat), depending on the cathode materials, anode materials, and electrolyte systems, this area has become a critical focus in lithium-ion battery safety research. Understanding these variations is essential, as they directly influence the battery’s thermal stability and risk of thermal runaway.
To investigate the thermal behavior of these reactions, Ph. Biensan et al. conducted a series of experiments using Differential Scanning Calorimetry (DSC). Their study systematically analyzed the exothermic reactions that occur within lithium-ion batteries under abuse conditions. The findings are summarized in Table, which outlines the heat release and temperature ranges associated with different material combinations and reaction stages.
|
Temperature Range (°C) |
Reaction |
Energy (J/g) |
Description |
|---|---|---|---|
|
110–150 |
LiₓC₆ + electrolyte |
350 |
Rupture of passivation layer |
|
130–180 |
Fusion separator P.E. |
–190 |
Endothermic |
|
160–190 |
Fusion separator P.P. |
–90 |
Endothermic |
|
180–500 |
600 |
Oxygen emission peak T ≈ 200°C |
|
|
220–500 |
Decomposition of Li₀.₄₅CoO₂ + electrolyte |
450 |
Oxygen emission peak T ≈ 230°C |
|
150–300 |
Decomposition of Li₀.₁Mn₂O₄ + electrolyte |
450 |
Oxygen emission T ≈ 300°C |
|
130–220 |
Solvents + LiPF₆ |
250 |
Low energy |
|
240–350 |
LiₓC₆/PVDF binder (rinsed) |
1500 |
Violent-propagation |
|
660 |
Fusion of aluminium |
–395 |
Endothermic |
Thermal Reactions Inside Lithium-Ion Batteries

Cathode Materials
On the cathode side, the primary thermal reactions involve the decomposition of active materials and the oxidation of organic solvents. Among them, the oxidation of organic solvents releases a significant amount of heat, which can potentially lead to thermal runaway of the battery.
Dahn et al. found in their study that Li₀.₅CoO₂ decomposes at elevated temperatures, forming LiCoO₂, Co₃O₄, and oxygen gas (O₂). Subsequently, Co₃O₄ further decomposes into CoO and O₂. The oxygen released from the cathode materials reacts with the organic solvents, generating CO₂ and H₂O. The following equations describe the high-temperature reaction processes between Li₀.₅CoO₂ and EC (ethylene carbonate) proposed by Dahn:
-
Eq.1:
Li₀.₅CoO₂ → 0.5LiCoO₂ + (1/6)Co₃O₄ + (1/6)O₂ -
Eq.2:
Co₃O₄ → 3CoO + (1/2)O₂ -
Eq.3:
(5/2)O₂ + C₃H₄O₃ → 3CO₂ + 2H₂O
At elevated temperatures, charged lithium nickel oxide (LiNiO₂) and lithium manganese oxide (LiMn₂O₄) also decompose and release oxygen. Their decomposition reactions are as follows:
-
Eq.4:
LiₓNiO₂ → LiₓNiO₂₋ₓ + (1/2 − x/2)O₂ -
Eq.5:
Mn₂O₄ + (2/5)C₃H₄O₃ → 2MnO + (6/5)CO₂ + (4/5)H₂O
Anode Materials
Currently, graphite-based materials are the mainstream anode materials, so safety research is mostly limited to these carbonaceous materials. On the anode side, the primary reactions include the decomposition of the Solid Electrolyte Interphase (SEI) layer, reduction of organic solvents, and reactions between LiC₆ and the binder.
Dahn et al. studied the thermal behavior of the anode using Accelerating Rate Calorimetry (ARC) and demonstrated that the SEI film on the anode surface begins to thermally decompose at approximately 100–130°C. They proposed the corresponding decomposition reaction equations as follows:
-
Eq.6:
(CH₂OCO₂Li)₂ → Li₂CO₃ + C₂H₄ + CO₂ + (2/1)O₂
-
Eq.7:
2Li + (CH₂OCO₂Li)₂ → 2Li₂CO₃ + C₂H₄
After the SEI film is compromised, the electrolyte directly contacts LiC₆, where the solvents are reduced to lithium alkyl carbonates and small gaseous molecules such as CO, CO₂, and C₂H₂, releasing a significant amount of heat.
If the temperature continues to rise, the anode active material will react with the binder. When polyvinylidene fluoride (PVDF) is used as the binder, it reacts with LiC₆ to form substances such as lithium fluoride (LiF). Ph. Biensan et al., through Differential Scanning Calorimetry (DSC), found that the intensity of this reaction is directly related to the presence of electrolyte, as shown in Figure. Without electrolyte, the reaction releases a large amount of heat; with electrolyte present, the reaction between LiC₆ and electrolyte releases more heat than the reaction with PVDF. The strength of this reaction depends on the amount of PVDF present.
Du Pasquire et al. discovered that replacing pure PVDF with a PVDF+HFP (hexafluoropropylene) mixture reduces the heat generated by the reaction between the binder and LiC₆ by 23%. H. Maleki et al. found that using phenol-formaldehyde (PF) resin as a binder results in almost negligible heat generation from the binder-LiC₆ reaction.
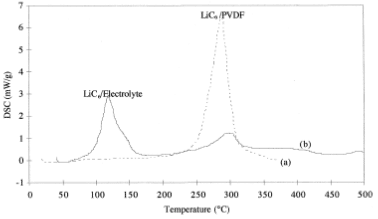
DSC curves of LiC₆/PVDF and LiC₆/Electrolyte
Safety Evaluation Criteria for Electrode Materials
The anode materials in lithium-ion batteries are primarily graphite-based, which have very similar structures. Therefore, their safety characteristics are almost linearly correlated with their specific surface area. For cathode materials, although safety is also related to specific surface area and particle size to some extent, the structural characteristics have a more significant impact on performance. Below are several methods mentioned in the literature for assessing the safety of cathode materials.
1. Oxygen Retention Capability (at High Temperature)
Clearly, the ability to retain oxygen in the charged state can be considered an important indicator of cathode material safety. Ph. Biensan and colleagues from Saft used Differential Scanning Calorimetry (DSC) to study the thermal behavior of various cathode materials. They found that lithium nickel oxide (LiNiO₂) has the poorest oxygen retention capability, starting to lose oxygen at around 200°C; lithium cobalt oxide (LiCoO₂) performs slightly better, losing oxygen at approximately 290°C; and spinel lithium manganese oxide (LiMn₂O₄) exhibits the best oxygen retention, with oxygen loss beginning only near 290°C. Modified lithium nickel oxide shows oxygen retention comparable to that of spinel lithium manganese oxide.
As for LiFePO₄, Whittingham et al. discovered that its charged state FePO₄ is an orthorhombic metastable phase that irreversibly transforms into a trigonal α-quartz-structured FePO₄ above 450°C. However, this transformation is very slow, and even at 500°C it is not complete. Within the tested temperature range, no oxygen loss was detected for LiFePO₄.
Based on this oxygen retention criterion, the safety ranking of cathode materials is:
LiFePO₄ > LiMn₂O₄ > LiCoO₂ > LiNiO₂
2. Thermal Stability
The thermal stability between charged cathode materials and the electrolyte solvent is an important criterion for assessing battery safety. Jiang et al. studied the thermal stability of charged LiNi₀.₁Co₀.₈Mn₀.₁O₂, LiFePO₄, and LiCoO₂ in contact with electrolyte solvents using an Accelerating Rate Calorimeter (ARC). Figure 1-2 shows the thermal behavior of these samples charged to 4.3 V when immersed in an EC/DMC solvent mixture.
It is evident that the ternary cathode material (LiNi₀.₁Co₀.₈Mn₀.₁O₂) exhibits better thermal stability than LiCoO₂, while LiFePO₄ shows significantly superior thermal stability compared to the ternary material. Jiang also confirmed that when simply heating the EC/DMC mixture alone, an endothermic peak appears around 320°C, indicating that FePO₄ likely did not undergo any reaction within the test temperature range.
Based on this thermal stability criterion, the materials rank as follows:
LiFePO₄ > LiNi₀.₁Co₀.₈Mn₀.₁O₂ > LiCoO₂
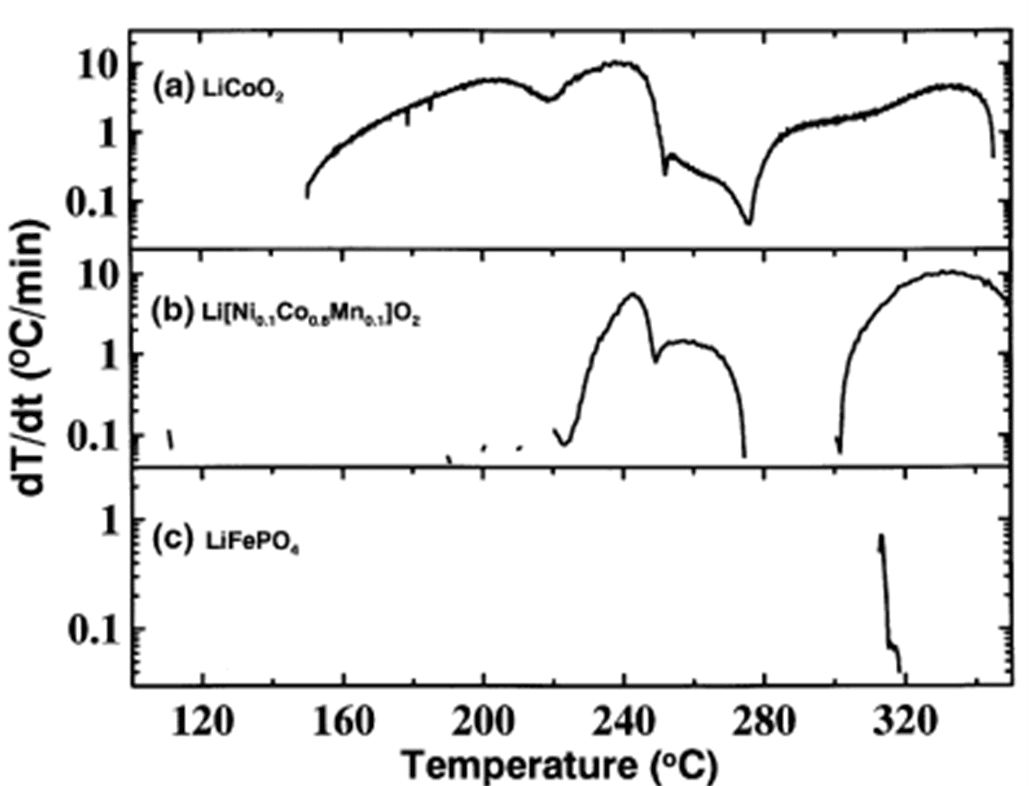
Comparison of the thermal stability of charged LiFePO₄, LiNi₀.₁Co₀.₈Mn₀.₁O₂, and LiCoO₂
3. Oxygen Retention Capability During Charging
The tendency of cathode materials to release oxygen during the charging process is also a critical factor affecting battery safety. Manthiram et al. reported that LiₓCoO₂ tends to lose lattice oxygen as lithium ions are extracted. In contrast, LiₓNi₀.₈₅Co₀.₁₅O₂ maintains its oxygen content as long as x ≥ 0.3. LiₓMn₂O₄ retains its oxygen stoichiometry (O:4) across a wide range of lithium content (0.15 ≤ x ≤ 1).
For LiₓFePO₄, oxygen loss does not occur even when x = 0. Additionally, its crystal structure is stabilized by PO₄ tetrahedral chains located between Fe–O layers. This structural feature minimizes volume change during lithium extraction, which contributes to the excellent cycling stability of LiFePO₄ and its low likelihood of structural collapse.
Based on this criterion, the cathode materials’ safety ranking is:
LiFePO₄ > LiMn₂O₄ > LiNi₀.₈₅Co₀.₁₅O₂ > LiCoO₂
4. Oxidation Capability Toward Electrolytes
The oxidative reactivity of cathode materials toward electrolyte solvents is another important parameter in evaluating safety. Dr. Weihe Kong and colleagues at the Institute of Physics, Chinese Academy of Sciences, analyzed the gas production of different cathode materials—LiCoO₂, LiMn₂O₄, and LiFePO₄—as an indicator of their oxidative strength.
Each battery was charged to 5.0 V, and the evolved gases were collected and analyzed via GC-MS. All three materials produced acetylene (C₂H₂), but CO₂—a typical oxidation byproduct of organic solvents—was not detected in the LiFePO₄-based cell. A small amount of CO₂ was found in the LiMn₂O₄ cell, while a large quantity of CO₂ was detected in the LiCoO₂ cell.
Since the oxidation of organic solvents involves dehydrogenation and oxygenation, the results suggest that:
LiFePO₄ > LiMn₂O₄ > LiCoO₂
in terms of safety, with LiFePO₄ showing the weakest oxidative reactivity toward electrolytes.
These evaluation methods for cathode material safety do not present any contradictions, which suggests that they are all valid criteria for assessing thermal safety. Based on the results discussed above, the general safety ranking of commonly used cathode materials is as follows:
LiFePO₄ > LiMn₂O₄ > LiNi₀.₈₅Co₀.₁₅O₂ > LiCoO₂ > LiNiO₂
For Li-Ni-Co-Mn-O layered cathode materials (NCMs), their safety performance depends on the ratio of nickel, cobalt, and manganese. Typically, high-nickel compositions are associated with higher energy density but lower thermal stability, while high-manganese compositions tend to exhibit better safety characteristics. Therefore, the safety of NCM materials is generally considered to lie between that of LiCoO₂ and LiMn₂O₄.
Conclusion
The materials used in lithium-ion batteries are fundamental to their overall safety and thermal stability. From cathode chemistry to electrolytes and binders, each component plays a critical role in minimizing risks such as overheating, fire, or thermal runaway. Based on several safety metrics—such as oxygen retention, thermal stability, and electrolyte reactivity—the relative safety ranking of mainstream cathode materials is: LiFePO₄ > LiMn₂O₄ > LiNi₀.₈₅Co₀.₁₅O₂ > LiCoO₂ > LiNiO₂.
At the same time, innovations in battery materials are reshaping the landscape. The development of solid-state electrolytes, improved binders, and next-generation cathode materials is making lithium-ion batteries safer and more reliable for everyday applications—from electric vehicles to industrial energy storage. Researchers worldwide are continuing to explore safer battery designs that protect people, reduce environmental impact, and meet the growing demands of modern industries.
With these ongoing advancements, the future of battery safety is bright, and material science will remain at the heart of this transformation.
FAQ
Why are lithium-ion batteries safer than older ones?
Lithium-ion batteries use better materials like stable cathodes. They also have separators with ceramic coatings. These features help stop overheating and short circuits. They are safer and work better than older batteries.
Can lithium-ion batteries catch fire easily?
No, they don’t catch fire often. But damage or too much heat can make them burn. Safer materials, like solid-state electrolytes, lower this risk a lot.
How do solid-state electrolytes make batteries safer?
Solid-state electrolytes use solid materials instead of flammable liquids. This removes fire risks and stops bad chemical reactions. They also block dendrites that cause short circuits.
Are lithium-ion batteries safe for electric cars?
Yes, they are safe for electric cars. Companies use materials like lithium iron phosphate to handle heat. These batteries are tested carefully to ensure safety.
What should you do if a lithium-ion battery gets too hot?
If a battery overheats, stop using it right away. Place it in a safe spot away from flammable things. Let it cool down and don’t touch it until an expert checks it.
See Also
Evaluating Soft Pack Versus Cylindrical Batteries For Drones
Selecting The Right Manufacturer For Your Drone Batteries